Preliminary Clinical Method Comparison of a Point-of-Care Platform for anti-Factor Xa in Pediatric Patients on Heparin Therapy
Read Further:
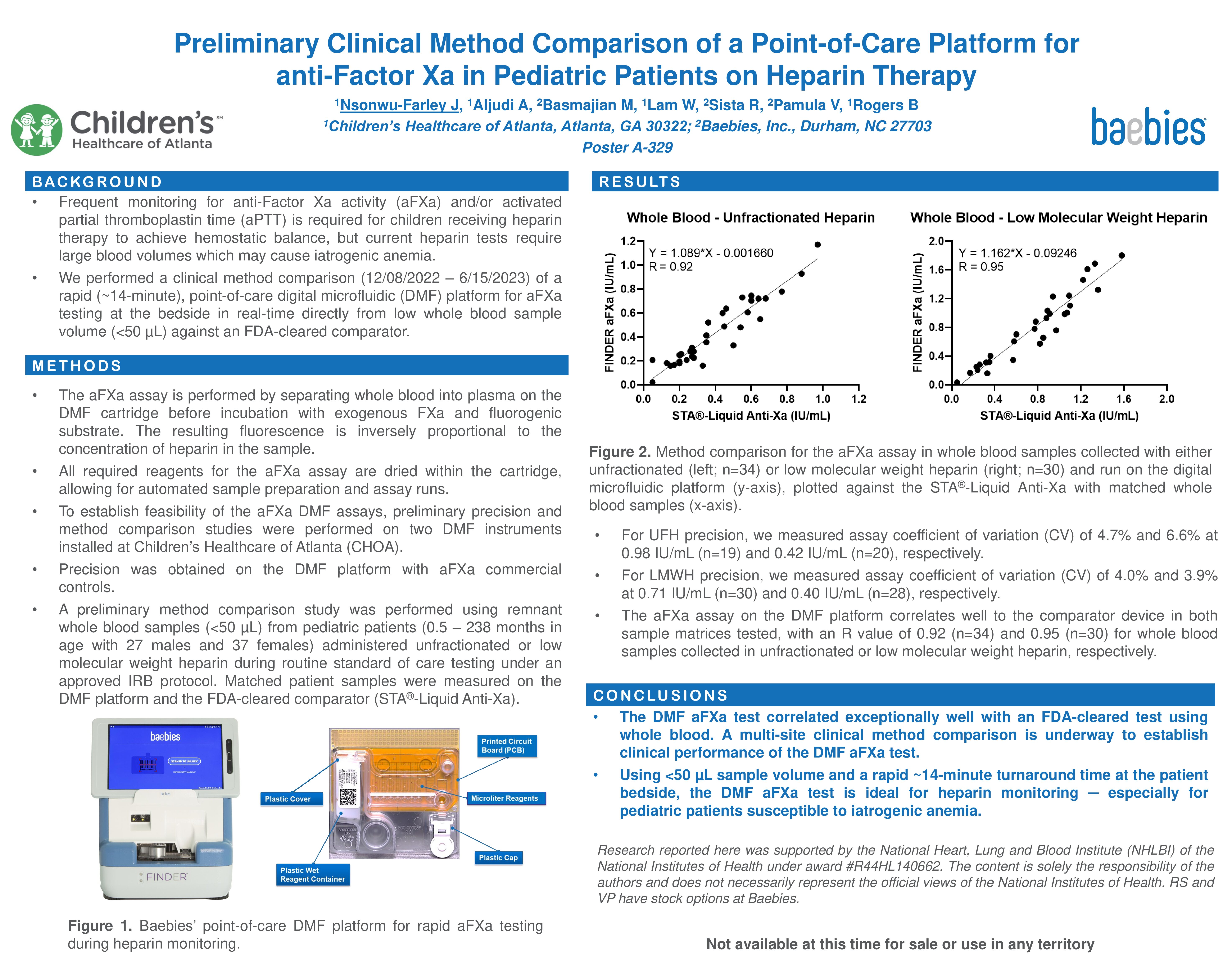
-
- Learn more about Digital Microfluidic Technology
- Baebies Receives FDA Breakthrough Device Designation for First Point-of-Care Heparin Monitoring Test
- Baebies Expands Its Vision: Any Test, Anywhere, Everyone™
- Feasibility of PT, aPTT, and Fibrinogen on a Multifunctional Digital Microfluidic Cartridge
- Novel Fluorometric Assay for Pediatric Anti-Factor Xa Testing: Minimizing Bilirubin and Hemolysis Interference in Whole Blood
- Learn more about Digital Microfluidic Technology
