Drugs with Warnings and Contraindications for G6PD Deficiency
 Glucose-6-phosphate dehydrogenase (G6PD) deficiency is among the most common human enzyme defects, present in about 400 million people across the globe1. Approximately 80,000 newborns are born with G6PD deficiency each year in the US alone2. G6PD deficiency is an inherited genetic disorder that affects red blood cells (RBCs)3. Individuals with G6PD deficiency have a reduced ability to defend RBCs against oxidative stress, resulting in hemolysis (the destruction of red blood cells) when exposed to certain triggers. Human migration and the concomitant increase in population diversity has increased the likelihood for clinicians to encounter G6PD deficiency outside of historically higher prevalence regions4,5. The disorder affects about 10%-13% African-American men6,7, and is also common in people from the Mediterranean region, Africa, or Asia8. Although the estimated percentage of Caucasians affected with G6PD deficiency is relatively low (0.2-0.3%)9, considering a US population of 334 million and current demographics10, roughly 700,000 Caucasian individuals are currently living with G6PD deficiency. Some individuals may not be aware of their G6PD status11.
Glucose-6-phosphate dehydrogenase (G6PD) deficiency is among the most common human enzyme defects, present in about 400 million people across the globe1. Approximately 80,000 newborns are born with G6PD deficiency each year in the US alone2. G6PD deficiency is an inherited genetic disorder that affects red blood cells (RBCs)3. Individuals with G6PD deficiency have a reduced ability to defend RBCs against oxidative stress, resulting in hemolysis (the destruction of red blood cells) when exposed to certain triggers. Human migration and the concomitant increase in population diversity has increased the likelihood for clinicians to encounter G6PD deficiency outside of historically higher prevalence regions4,5. The disorder affects about 10%-13% African-American men6,7, and is also common in people from the Mediterranean region, Africa, or Asia8. Although the estimated percentage of Caucasians affected with G6PD deficiency is relatively low (0.2-0.3%)9, considering a US population of 334 million and current demographics10, roughly 700,000 Caucasian individuals are currently living with G6PD deficiency. Some individuals may not be aware of their G6PD status11.
Compounds that present a risk to G6PD-deficient individuals include certain foods such as fava beans12, some chemicals such as naphthalene found in moth balls13 and several medicines. In fact, early insight into the biology of G6PD deficiency came from research on hemolysis caused by antimalarial drugs observed only in certain populations14. As tabulated by the G6PD deficiency association, 143 drug compounds are stratified as high, medium, and low risk to those with G6PD deficiency15. A recent search for “glucose-6-phosphate dehydrogenase (G6PD) deficiency” on the FDA’s Drug Product Labelling Website, yields 1 result found in the “Boxed Warning” section (Rasburicase), 13 results found in the “Contraindications” section, and 225 results found in the “Warning and Precautions” section of drug labelling16 . While the actual number of unique compounds from this text search is smaller; different drug forms and producers contribute to the number of search results with “glucose-6-phosphate dehydrogenase (G6PD) deficiency” in the labelling. To manage their condition, individuals with G6PD deficiency need to be aware of drugs that can potentially exacerbate their condition.
Drugs with contraindications for G6PD Deficiency
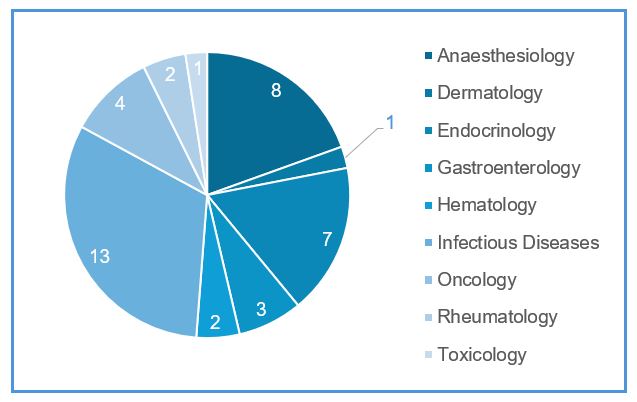
In this blog, we will discuss multiple drugs that have warnings or contraindications for individuals with G6PD deficiency. When G6PD-deficient individuals are exposed to triggers such as these medications, it can lead to a breakdown of red blood cells, resulting in hemolysis and associated symptoms like anemia, jaundice, and dark urine. The figure below shows the distribution of all the drugs in different therapeutic areas that have either a black box warning or contra-indication for G6PD deficiency.
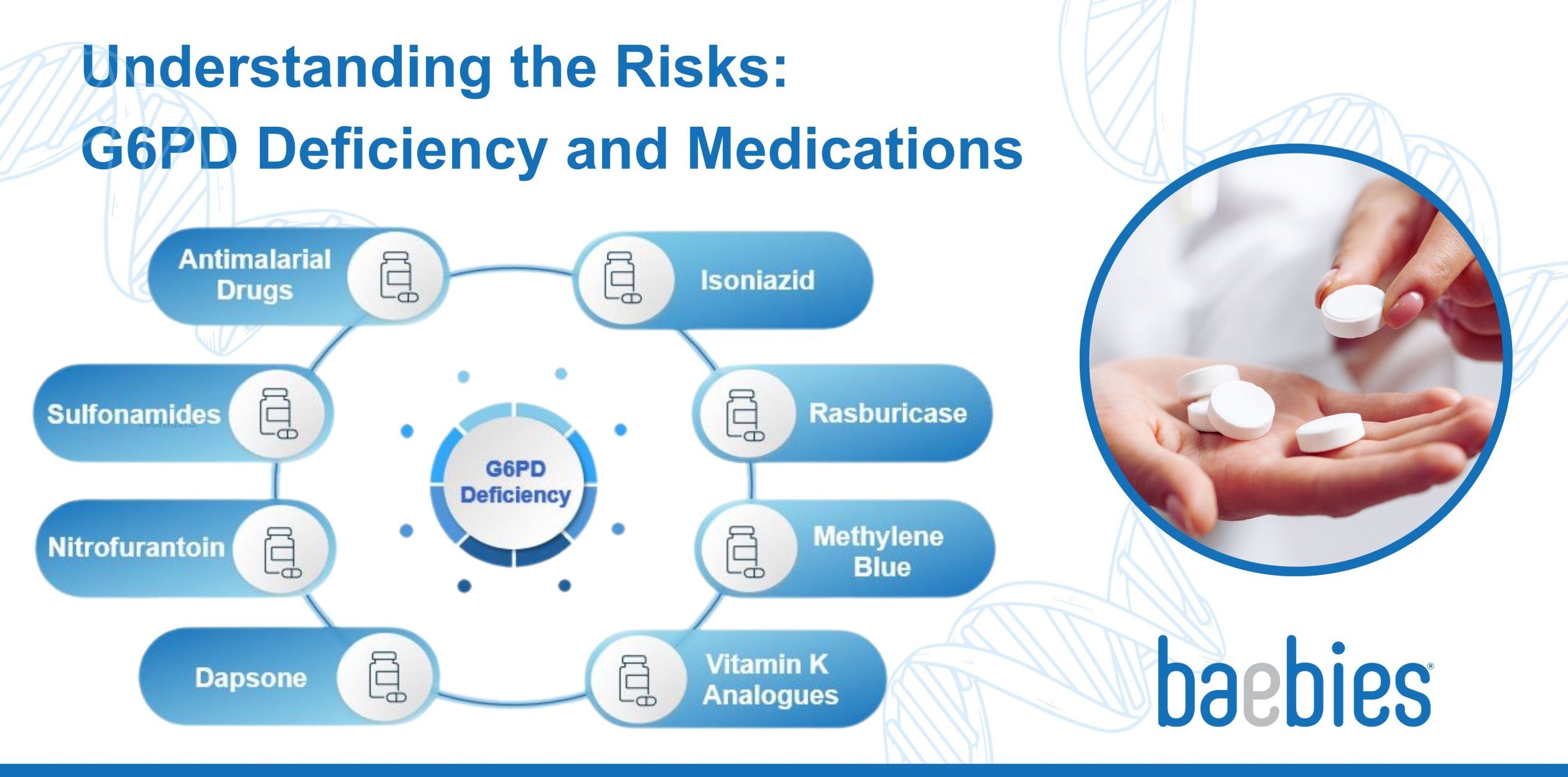
Below is a list of the different classes of drugs with warnings or contra-indications for G6PD Deficiency
- Antimalarial Drugs: Some antimalarial medications, like primaquine and tafenoquine, are known to cause severe hemolysis in individuals with G6PD deficiency. They are often avoided in these patients, although they may be prescribed under strict medical supervision17.
- Sulfonamides: Drugs in this class, such as sulfamethoxazole, can trigger hemolysis in some G6PD-deficient individuals18,19. They are commonly used in antibiotics with a brand name of Bactrim and should be used with caution20 or avoided in such cases. A total of 5 million prescriptions of Bactrim are given each year in the USA21.
- Nitrofurantoin: This antibiotic is used to treat urinary tract infections and may cause hemolysis in individuals with G6PD deficiency22. They are available with brand names Furadantin or Ivadantin with over 4 million prescriptions given each year in the USA23.
- Dapsone: With over 250,000 prescriptions per year in the USA24, Dapsone is used to treat various skin conditions and is known to cause severe hemolysis in G6PD-deficient individuals25.
- Isoniazid: Used in the treatment of tuberculosis, isoniazid can cause drug-induced hemolysis26 and should be used cautiously in G6PD-deficient patients.
- Rasburicase: This drug is used to prevent and treat high uric acid levels in cancer patients receiving chemotherapy. Rasburicase can lead to hemolysis in individuals with G6PD deficiency27 and should be avoided.
- Methylene Blue: Used as a dye in certain medical procedures and as a treatment for methemoglobinemia, methylene blue can cause hemolysis in individuals with G6PD deficiency28.
- Vitamin K Analogues: Some vitamin K analogues, such as menadione (Vitamin K3), have been associated with hemolysis in individuals with G6PD deficiency29 and should be avoided in favor of other vitamin K formulations30.
G6PD testing is essential before administering drugs
It is important to note that the severity of the reaction to these drugs can vary among individuals with G6PD deficiency. Some individuals may tolerate certain medications better than others. However, it’s generally advisable for healthcare providers to exercise caution when prescribing these drugs or consider alternative treatments. Some of the drugs that are mentioned above are prescribed without prior diagnosis for G6PD deficiency with the potential to result in adverse reactions, see case report in31.
Additionally, many of these compounds can potentially transfer from mother to baby through breast milk32. This is known as drug excretion into breast milk, and it can vary depending on the specific drug, and the physiological characteristics of the mother and baby. It is critical to test individuals for G6PD deficiency before administering certain therapies to avoid severe adverse outcomes. For newborns, it is even more critical to ensure G6PD testing is performed before discharge to ensure proper treatment decisions are made both for the newborn and the mother with alternative drug treatments as required.
Conclusion
Individuals with G6PD deficiency must be vigilant about drugs they take to avoid potential complications and life-threatening hemolysis. It is essential for patients to inform their healthcare providers about their G6PD deficiency, and for healthcare providers to carefully consider the risks and benefits of medications when treating such individuals. It is critical that G6PD testing is performed before administering these therapies with contraindications and black box warnings. Additionally, research into G6PD deficiency and its interactions with medications continues, and new findings may lead to updates in treatment guidelines.
References
-
- Mason, Philip J. “New insights into G6PD deficiency.” British journal of haematology 94.4 (1996): 585-591
- Vidavalur, Ramesh, and Vinod K. Bhutani. “Estimates of Racial Diversity of National (USA) glucose-6-phosphate Dehydrogenase Deficiency (G6PDd) Prevalence Among Newborns.” Pediatrics 149.1 Meeting Abstracts February 2022 (2022): 670-670
- https://medlineplus.gov/genetics/condition/glucose-6-phosphate-dehydrogenase-deficiency/
- Van den Heuvel, E. A. L., et al. “A rare disorder or not? How a child with jaundice changed a nationwide regimen in the Netherlands.” Journal of Community Genetics 8.4 (2017): 335
- Kristensen, Line, et al. “A greater awareness of children with glucose‐6‐phosphate dehydrogenase deficiency is imperative in western countries.” Acta Paediatrica 110.6 (2021): 1935-1941
- Kaplan, Michael, et al. “Neonatal hyperbilirubinemia in African American males: the importance of glucose-6-phosphate dehydrogenase deficiency.” The Journal of Pediatrics 149.1 (2006): 83-88
- Leung-Pineda, Van, Elizabeth P. Weinzierl, and Beverly B. Rogers. “Preliminary Investigation into the Prevalence of G6PD Deficiency in a Pediatric African American Population Using a Near-Patient Diagnostic Platform.” Diagnostics 13.24 (2023): 3647
- Nkhoma, Ella T., et al. “The global prevalence of glucose-6-phosphate dehydrogenase deficiency: a systematic review and meta-analysis.” Blood Cells, Molecules, and Diseases 42.3 (2009): 267-278
- Chinevere, Troy D., et al. “Prevalence of glucose-6-phosphate dehydrogenase deficiency in US Army personnel.” Military Medicine 171.9 (2006): 905-907
- https://www.census.gov/quickfacts/
- https://baebies.com/jessicas-story-a-remarkable-fight-for-answers-with-g6pd-deficiency/
- Luzzatto, Lucio, and Paolo Arese. “Favism and glucose-6-phosphate dehydrogenase deficiency.” New England Journal of Medicine 378.1 (2018): 60-71
- Begum, Afsana. “Naphthalene Poisoning in a Young Glucose 6 Phosphate Dehydrogenase Deficient Patient.” Bangladesh Journal of Medicine (2023): 218-218
- Carson, Paul E., et al. “Enzymatic deficiency in primaquine-sensitive erythrocytes.” Science 124.3220 (1956): 484-485
- https://www.g6pd.org/en/G6PDDeficiency/SafeUnsafe/drugs-official-list
- https://nctr-crs.fda.gov/fdalabel/ui/search
- https://www.who.int/publications/i/item/9789241514286
- Chisholm-Burns MA, Patanwala AE, Spivey CA. “Aseptic meningitis, hemolytic anemia, hepatitis, and orthostatic hypotension in a patient treated with trimethoprim-sulfamethoxazole.” Am J Health Syst Pharm. 2010;67(2):123-7. doi:10.2146/ajhp080558. PMID: 20065266
- Reinke CM, Thomas JK, Graves AH. “Apparent hemolysis in an AIDS patient receiving trimethoprim/sulfamethoxazole: case report and literature review.” J Pharm Technol. 1995;11(6):256-62; quiz 293-5. doi:10.1177/875512259501100607. PMID: 10157546
- Lu, Y. W., and T. C. Chen. “Use of Trimethoprim-Sulfamethoxazole in Patient with G6PD Deficiency for Treating Pneumocystis Jirovecii Pneumonia.” Clin Case Rep Int. 2019;3:1119
- https://clincalc.com/DrugStats/Drugs/SulfamethoxazoleTrimethoprim
- Recht, Judith, et al. “Nitrofurantoin and glucose-6-phosphate dehydrogenase deficiency: a safety review.” JAC-Antimicrobial Resistance 4.3 (2022): dlac045
- https://clincalc.com/DrugStats/Drugs/Nitrofurantoin
- https://clincalc.com/DrugStats/Drugs/Dapsone
- Pamba, Allan, et al. “Clinical spectrum and severity of hemolytic anemia in glucose 6-phosphate dehydrogenase–deficient children receiving dapsone.” Blood 120.20 (2012): 4123-4133
- Youngster, Ilan, et al. “Medications and glucose-6-phosphate dehydrogenase deficiency: an evidence-based review.” Drug Safety 33 (2010): 713-726
- Hammami, M. Bakri, et al. “Rasburicase-induced hemolytic anemia and methemoglobinemia: A systematic review of current reports.” Annals of Hematology (2023): 1-13
- Jack Clifton II, and Jerrold B. Leikin. “Methylene blue.” American Journal of Therapeutics 10.4 (2003): 289-291
- Lucey, Jerold F., and Robert G. Dolan. “Hyperbilirubinemia of newborn infants associated with the parenteral administration of a vitamin K analogue to the mothers.” Pediatrics 23.3 (1959): 553-560
- Kaplan, Michael, et al. “Effect of vitamin K1 on glucose-6-phosphate dehydrogenase deficient neonatal erythrocytes in vitro.” Archives of Disease in Childhood. Fetal and Neonatal Edition 79.3 (1998): F218
- Foltz, Lynda M., et al. “Recognition and management of methemoglobinemia and hemolysis in a G6PD‐deficient patient on experimental anticancer drug Triapine.” American Journal of Hematology 81.3 (2006): 210-211
- Corchia C, Balata A, Meloni GF, Meloni T. “Favism in a female newborn infant whose mother ingested fava beans before delivery.” J Pediatr 1995;127:807-8
*All reference websites were accessed as of date 1.26.2024
